THE LASER
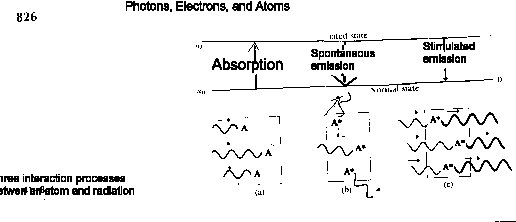
If the energy difference between the normal and the first excited state of an atom is E, the atom is capable of absorbing a Photon whose frequency f is given by the Planck equation E =hf . The absorption of a photon by a normal atom is depicted schematically in Fig. 41-8a. After absorbing the photon, the atom becomes an excited atom A*. A short time later, spontaneous emission takes place and the excited atom becomes normal again by emitting a photon of the same frequency as that which was originally absorbed by in a random direction and with a random phase, as shown in Fig. 41-8b.

There is also a third process, first proposed by Einstein, called simulated emission, shown schematically in Fig. 41-8c. Stimulated emission takes placed shen a photon encounters an excited atom and forces it to emit another photon of the some frequency, in the same direction, and in the same phase. The two photons go off together as coherent radiation.
Consider an absorption cell containing a large number of atoms of the type depicted in Fig. 41-8. In the absence of an external beam of radiation, most of the atoms are in the ground state; there are only a few excited atoms present in the cell. The ratio of the number nE of excited atoms to the number no of normal atoms is extremely small.
Now suppose a beam of radiation is sent through the cell with frequency f corresponding to the energy difference E. The ratio of the numbers nE and NO, that is, he ratio of the populations of the energy levels, is increased, Since the population of the normal state was so much larger than that of the excited state, an enormously intense beam of light would be required to increase the population of the excited state to a value comparable to or greater than that of the normal state. Therefore, the rate t which energy is extracted from the beam by absorption of normal atoms far outweighs the rate at which energy is added to the beam by stimulated emission of excited atoms.
If a condition can be created in which NE is substantially increased compared to the normal equilibrium value, creating a condition known as population inversion, the rate of energy radiation by stimulated emission may exceed the rate of abortion. The system then acts as a source of radiation with photon energy E. Furthermore, since the photons are the result of stimulated emission, they all have the same frequency, phase, polarization, and direction. The resulting radiation id therefore very much more coherent than light form ordinary sources, in which the emissions of individual atoms are not coordinated.
The necessary population inversion can be achieved a variety of ways. As an example we consider the helium-neon laser, a simple, inexpensive laser available in many undergraduate laboratories. A mixture of helium and neon, each typically at a pressure of the order of 10^2 Pa (or 10^-3 atm), is sealed in a glass enclosure provided with two electrodes. When a sufficiently high voltage is applied, a glow discharge occurs. Collisions between ionized atoms and electrons carrying the discharge current excite atoms to various energy states.
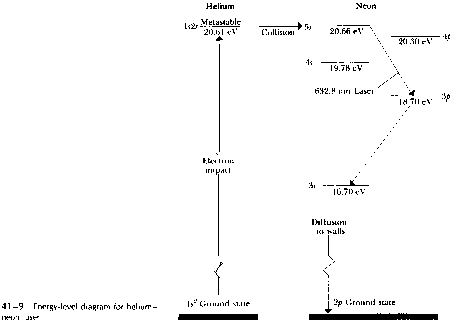
Figure 41-9 sows an energy-level diagram for the system. The notation used to label the various energy levels, such as 1s2s or 5s, will be discussed in Section 43-1, and need not concern us here. Helium atoms excited to the 1s2s state cannot return to the ground state by emitting a 20.61-eV photon, as might be expected, because both the states have zero total angular momentum, while a photon must carry away at least one unit (h/2p) of angular momentum. Such a state, in which radioactive decay is impossible, is called a metastable state.
The helium atoms can, however, lose energy by energy -exchange collisions with neon atoms initially in the ground state. A 1s2s helium atom, with its internal energy of 20.61 eV and a little additional kinetic energy, can collide either a neon atom in the ground state, exciting it to the 5s excited state at 20.66 eV and leaving the gelium atom in the 1s2 ground state. Thus, we hae the necessary mechanism to a population inversion in neon, with the population in the 5s state substantially enhanced. Stimulated emission from this state then results in the emission of highly coherent light at 632.8 nm, as shown on the dia gram. In practice the beam is sent back and forth through the gas many times by a pair of parallel mirrors, so as to stimulate emission from as many excited atoms as possible.One of the mirrors is partially transparent. so a portion of the beam emerges as an external beam.
The net effect of all the processes taking place in a laser tube is a beam of radiation that is (1) very expensive, (2) almost perfectly parallel, (3) almost monochromatic, and (4)spatially coherent at all points within a given cross section. To understand this fourth characteristic, we recall the simple soluble-slit interference experiment. A mercury arc placed directly behind the double slit would not five rise to interference fringes because the light issuing from the two slits would come from different points of the arc and would not retain a constant phase relationship. In the use of the usual laboratory arc-lamp sources, it is necessary to use the light from avert small portion of the source to illuminate the double slit. The slightly diverging beam from a laser, however, may be allowed to fall directly on a double slit ( or other inter ferometer) because the light rays from any two points of a cross section are in phase, and are said to exhibit "spatial coherence."
In recent years lasers have found a wide variety of practical applications. The high intensity of a laser beam makes it a convenient drill. A very small hole can be drilled in a diamond for use as a die in drawing very small-diameter wire. The ability of a laser beam to travel long distances without appreciable spreading makes it a very useful tool of surveyors, especially in situations where great precision is required over long distances, as in the case of a long tunnel drilled from both ends.ed retina; and the resulting
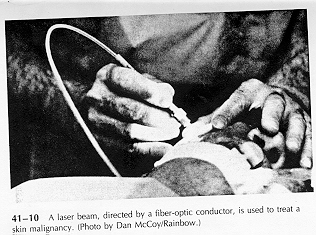
Lasers are finding increasing application in medical science. A laser can produce a very narrow beam with extremely high intensity, high enough to vaporize anything in itspath. This poperty is used in the treatment of a detached retina, and the resulting scar tissue "welds" the retin back to the choroid from which it has become detached. Laser beams are also used in surgery; blood vessels cut by the beam tend to seal temselves off, making it easier to control bleeding. The use of laser radiation in the treatment of skin concer is an actrive area of research, as shown in Fig. 41-10.